Documenting Standard Operating Procedures in 2025: A Life Sciences Guide
- Dr. Sarah King

- Jul 4, 2025
- 4 min read

Research and development (R&D) and quality assurance (QA) are defined by innovation, precision, and, perhaps above all, compliance. Whether developing a new pharmaceutical product, refining a medical device, or ensuring the safety of a food product, organizations must navigate a maze of regulations and standards.
At the heart of this process lies the Standard Operating Procedure (SOP). This article breaks down the SOP, why they matter, how they’re structured, and how organizations can leverage them for regulatory and operational success.

What Are SOPs and Why Do They Matter?
An SOP is a detailed, written instruction designed to achieve uniformity in the performance of a specific function. In the context of research and QA, SOPs serve as the backbone of reliable, reproducible processes, ensuring that every step, from sample preparation to data analysis, is performed consistently, regardless of who is carrying out the task.
Purpose and Importance of Documenting Standard Operating Procedures for Regulatory Compliance
SOPs are not just about efficiency; they are essential for meeting the rigorous requirements set by regulatory bodies such as the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonisation (ICH). These agencies, along with Good Practice (GxP) standards, require organizations to document their processes in a way that guarantees traceability, accountability, and reproducibility. Without robust SOPs, organizations risk failed audits, regulatory penalties, and, ultimately, compromised patient safety.
Essential Components and Format of SOPs
A well-constructed SOP typically includes:
Title Page: Document title, unique identifier, version number, and approval signatures.
Purpose and Scope: Why the SOP exists and which processes or departments it covers.
Responsibilities: The person/people responsible for executing, reviewing, and approving the SOP.
Procedure: Step-by-step instructions including safety considerations and critical control points.
References and Appendices: Supporting documents, forms, and related SOPs.
Consistency is important. SOPs should use clear headings, numbered steps, bullet points, and standardized terminology. Visual aids such as flowcharts or diagrams can enhance understanding and reduce errors. Avoid vague language like “as needed”, excessive jargon, or ambiguous instructions. SOPs should be technical, actionable, concise, and accessible to all intended users.
The 4 P’s Framework
A practical approach to SOP creation is the “4 P’s”:
Purpose: Why is this SOP needed?
Process: What steps are involved?
People: Who is responsible?
Proof: How is compliance demonstrated (records, logs, signatures)?
Regulatory Requirements and Compliance Considerations
SOPs are shaped by a complex web of regulations:
FDA 21 CFR Part 11 (electronic records and signatures)
EMA and ICH Q10 (Pharmaceutical Quality Systems)
ISO 9001 (quality management systems)
GxP (Good Laboratory, Clinical, and Manufacturing Practices)
Recent years have seen increased scrutiny of data integrity, electronic documentation, and risk-based approaches to quality management. Agencies now expect organizations to demonstrate not just the existence of SOPs, but their effective implementation. SOPs should be written and maintained with audits in mind. This means clear version control, documented training records, and evidence of regular review and updates. Furthermore, integrating risk assessment into SOP development helps prioritize critical processes and allocate resources where they are most needed.

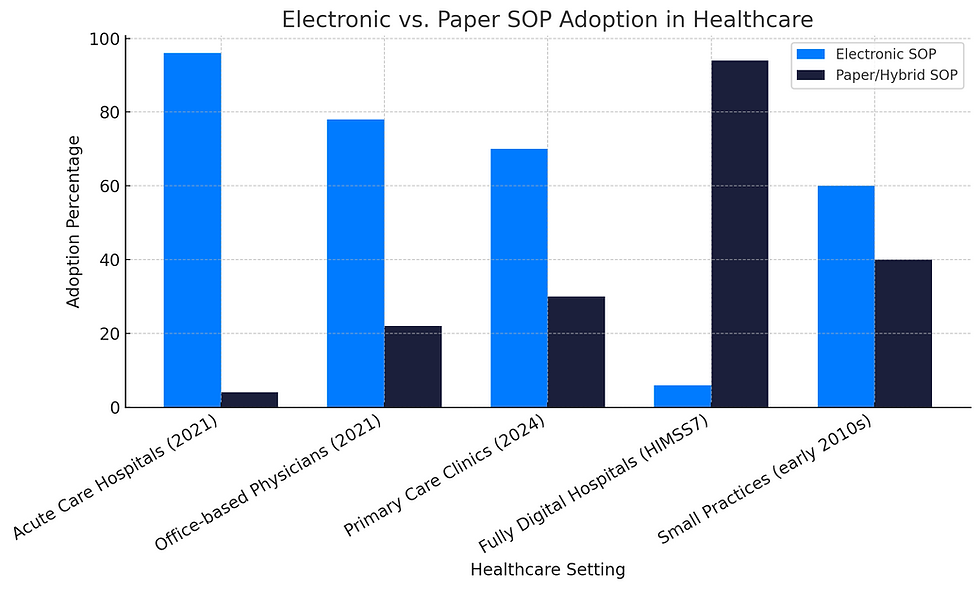
Electronic SOP Systems vs. Paper-Based Documentation
Electronic SOP management systems provide version control, search functionality, automated reminders, and secure access. They reduce the risk of lost or outdated documents and streamline the audit process. Modern SOP systems can integrate with project management tools and learning management systems to ensure seamless training and real-time compliance tracking.
SOP Development, Review, and Approval
There are a number of steps involved in documenting Standard Operating Procedures and their creation process:
Identify the Need: What process requires standardization?
Draft the SOP: Collaborate with process owners and end-users.
Internal Review: Circulate for feedback from users across the organization.
Approval: Obtain sign-off from QA and management.
Training: Ensure all relevant staff are trained before implementation.
Best practices for version control include assigning unique identifiers, tracking revisions, and archiving obsolete versions. A change log should be maintained, detailing what was updated and the reasons behind the updates. To ensure the SOP is practical and compliant, it is important to involve representatives from QA, R&D, operations, and regulatory affairs. Furthermore, audit trails should be clear and document every stage of the review and approval process. This enables full transparency and accountability.

SOP Implementation and Staff Training
Effective SOP implementation begins with clear communication of the purpose and benefits of new procedures, using kick-off meetings, internal newsletters, and digital platforms to engage staff and foster buy-in. Tailored training programs are essential. Developing role-specific modules that incorporate quizzes, hands-on demonstrations, and competency assessments ensures that all team members understand and can apply the SOPs in their daily work.
Ongoing monitoring through audits and regular feedback helps track adherence, quickly identifying and addressing any barriers to compliance. Overcoming common adoption challenges, such as resistance to change and information overload, requires transparent communication, visible leadership support, and a commitment to continuous training and improvement.
Maintenance, Review Cycles, and Continuous Improvement
Effective maintenance of SOPs relies on establishing regular review cycles, typically every one to three years, or initiating updates in response to audits, incidents, or regulatory changes. Incorporating feedback from audit findings, user input, and performance metrics ensures that SOP revisions are relevant and impactful. Measuring the effectiveness of SOPs by tracking deviation rates, audit outcomes, and training completion helps organizations assess their real-world impact and identify areas for improvement.
To maximize efficiency and compliance, it is essential to align SOP maintenance with the organization’s broader quality management system (QMS), ensuring that updates are systematic, traceable, and fully integrated into overall quality processes.
Conclusion
SOPs are far more than regulatory paperwork; they are the scaffolding upon which quality, safety, and innovation are built. By following best practices in SOP development, implementation, and maintenance, organizations can ensure compliance, reduce risk, and foster a culture of continuous improvement. As digital transformation accelerates, electronic SOP systems will play an ever greater role in streamlining processes and ensuring audit readiness. For organizations ready to take the next step, a wealth of templates and resources is available to jumpstart SOP excellence, laying the groundwork for R&D, regulatory, and commercial success in an increasingly complex world.
At Co-Labb, our PhD-educated writers bring extensive experience in regulatory writing. Whether you're interested in documenting standard operating procedures, preparing a clinical study report, or any other regulatory documentation, we’re here to support you at every stage. Contact us today to ensure your submissions are compliant, clear, and delivered to the highest standard.